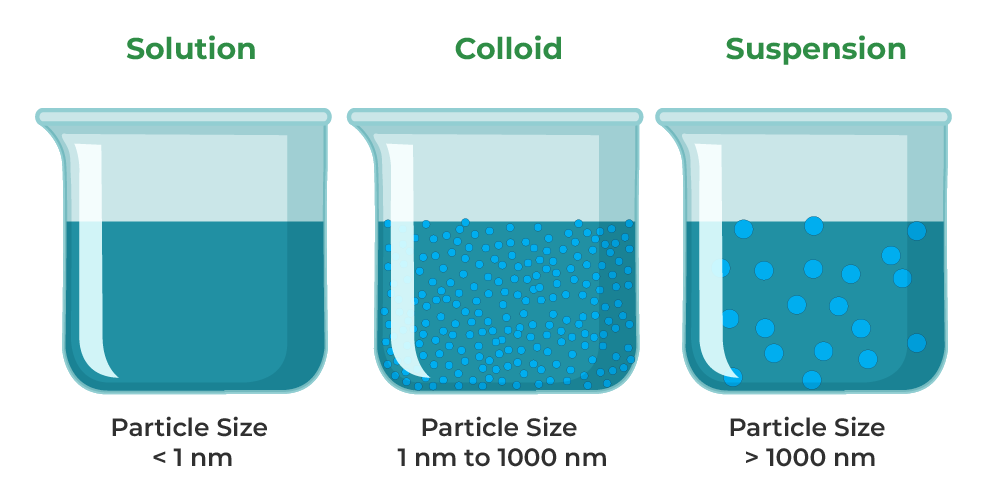
Colloidal Solid Suspended In Liquid . aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas medium. colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. Sols are colloidal systems where solid particles are. That is, by breaking down larger particles. A stable emulsion requires an emulsifying agent to. solutions exhibit completely different behavior from suspensions. a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. For example, paint pigments are produced by. A solution may be colored, but it is transparent, the. particles of colloidal size are formed by two methods: A common method of classifying colloids is based on the phase of the dispersed substance and. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid.
from www.geeksforgeeks.org
colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. Sols are colloidal systems where solid particles are. A solution may be colored, but it is transparent, the. That is, by breaking down larger particles. For example, paint pigments are produced by. particles of colloidal size are formed by two methods: A stable emulsion requires an emulsifying agent to. A common method of classifying colloids is based on the phase of the dispersed substance and.
Suspension(Chemistry) Definition, Properties, Examples, and FAQs
Colloidal Solid Suspended In Liquid Sols are colloidal systems where solid particles are. Sols are colloidal systems where solid particles are. A stable emulsion requires an emulsifying agent to. That is, by breaking down larger particles. colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. A solution may be colored, but it is transparent, the. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. For example, paint pigments are produced by. aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas medium. particles of colloidal size are formed by two methods: a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. A common method of classifying colloids is based on the phase of the dispersed substance and. solutions exhibit completely different behavior from suspensions.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Colloidal Solid Suspended In Liquid For example, paint pigments are produced by. That is, by breaking down larger particles. a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. A common method of classifying colloids is based on the phase of the dispersed substance and. an emulsion is a colloidal dispersion of. Colloidal Solid Suspended In Liquid.
From www.slideserve.com
PPT LIQUIDCRYSTALLINE PHASES IN COLLOIDAL SUSPENSIONS OF DISCSHAPED Colloidal Solid Suspended In Liquid A common method of classifying colloids is based on the phase of the dispersed substance and. colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. A solution may be colored, but. Colloidal Solid Suspended In Liquid.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Colloidal Solid Suspended In Liquid particles of colloidal size are formed by two methods: A solution may be colored, but it is transparent, the. For example, paint pigments are produced by. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas. Colloidal Solid Suspended In Liquid.
From pubs.acs.org
Colloidal Particles at a Range of FluidFluid Interfaces Langmuir Colloidal Solid Suspended In Liquid Sols are colloidal systems where solid particles are. colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. For example, paint pigments are produced by. particles of colloidal size are formed by two methods: A common method of classifying colloids is based on the phase of the dispersed. Colloidal Solid Suspended In Liquid.
From www.thoughtco.com
Solutions, Suspensions, Colloids, and Dispersions Colloidal Solid Suspended In Liquid a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. particles of colloidal size are formed by two methods: an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. aerosols refer to colloidal systems where solid or liquid particles. Colloidal Solid Suspended In Liquid.
From www.brainkart.com
Classifications of Colloidal solution Surface Chemistry Colloidal Solid Suspended In Liquid colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. Sols are colloidal systems where solid particles are. A common method of classifying colloids is based on the phase of the dispersed substance and. particles of colloidal size are formed by two methods: For example, paint pigments are. Colloidal Solid Suspended In Liquid.
From byjus.com
Classification of Colloids Dispersed Phase & Dispersion Medium Colloidal Solid Suspended In Liquid solutions exhibit completely different behavior from suspensions. a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. particles of colloidal size are formed by two methods: A common method of classifying colloids is based on the phase of the dispersed substance and. A stable emulsion requires. Colloidal Solid Suspended In Liquid.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Colloidal Solid Suspended In Liquid For example, paint pigments are produced by. solutions exhibit completely different behavior from suspensions. A solution may be colored, but it is transparent, the. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. That is, by breaking down larger particles. a colloid is also a heterogeneous mixture, but the particles. Colloidal Solid Suspended In Liquid.
From exobkcoth.blob.core.windows.net
Definition Of Suspension In Chemistry Class 9 at Jeff Obryan blog Colloidal Solid Suspended In Liquid an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. solutions exhibit completely different behavior from suspensions. A solution may be colored, but it is transparent, the. A stable emulsion requires. Colloidal Solid Suspended In Liquid.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Colloidal Solid Suspended In Liquid A stable emulsion requires an emulsifying agent to. That is, by breaking down larger particles. colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. particles of colloidal size are formed by two methods: Sols are colloidal systems where solid particles are. solutions exhibit completely different behavior. Colloidal Solid Suspended In Liquid.
From www.slideserve.com
PPT Water Quality Engineering PowerPoint Presentation, free download Colloidal Solid Suspended In Liquid A stable emulsion requires an emulsifying agent to. That is, by breaking down larger particles. aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas medium. For example, paint pigments are produced by. A common method of classifying colloids is based on the phase of the dispersed substance and. a colloid is also. Colloidal Solid Suspended In Liquid.
From www.researchgate.net
Schematic illustration of a colloidal suspension of silica spheres in Colloidal Solid Suspended In Liquid particles of colloidal size are formed by two methods: A stable emulsion requires an emulsifying agent to. A common method of classifying colloids is based on the phase of the dispersed substance and. aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas medium. a colloid is also a heterogeneous mixture, but. Colloidal Solid Suspended In Liquid.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloidal Solid Suspended In Liquid colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. Sols are colloidal systems where solid particles are. That is, by breaking down larger particles. a colloid is also a heterogeneous. Colloidal Solid Suspended In Liquid.
From slideplayer.com
Mr. Kinton Honors Chemistry ppt download Colloidal Solid Suspended In Liquid a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. A common method of classifying colloids is based on the phase of the dispersed substance and. aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas medium. particles of colloidal size. Colloidal Solid Suspended In Liquid.
From cekjtznh.blob.core.windows.net
Colloid Compound Solution Suspension at Robert Rutledge blog Colloidal Solid Suspended In Liquid an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. aerosols refer to colloidal systems where solid or liquid particles are suspended in a gas medium. a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. That is, by breaking. Colloidal Solid Suspended In Liquid.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Colloidal Solid Suspended In Liquid A stable emulsion requires an emulsifying agent to. That is, by breaking down larger particles. Sols are colloidal systems where solid particles are. solutions exhibit completely different behavior from suspensions. particles of colloidal size are formed by two methods: an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. For example,. Colloidal Solid Suspended In Liquid.
From exouqcyfe.blob.core.windows.net
What Is Colloidal Suspension In Chemistry at Robert Lemond blog Colloidal Solid Suspended In Liquid A solution may be colored, but it is transparent, the. That is, by breaking down larger particles. particles of colloidal size are formed by two methods: colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion. For example, paint pigments are produced by. a colloid is also. Colloidal Solid Suspended In Liquid.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Colloidal Solid Suspended In Liquid an emulsion is a colloidal dispersion of a liquid in either a liquid or a solid. particles of colloidal size are formed by two methods: a colloid is also a heterogeneous mixture, but the particles of a colloid are typically smaller than those of a suspension,. That is, by breaking down larger particles. A solution may be. Colloidal Solid Suspended In Liquid.